Trina Schroer
Professor
Contact Information
- [email protected]
- 203 Biology East
- 410-516-5373 | Lab 410-516-5374
Research Interests: Microtubule-based motor proteins and their roles in cellular architecture
Education: PhD, University of California, San Francisco
Trina Schroer is a professor of biology whose primary research focus is microtubule-based motor proteins and their roles in cellular architecture. She earned her BS from Stanford University, her PhD from the University of California, San Francisco, and did her postdoctoral work at Washington University School of Medicine.
Work in the Schroer lab addresses questions of cell motility: movement within the cell and movement of the cell as a whole. Our focus is on the microtubule cytoskeleton, the motors that power movement, and the cargo structures and molecules with which motors interact.
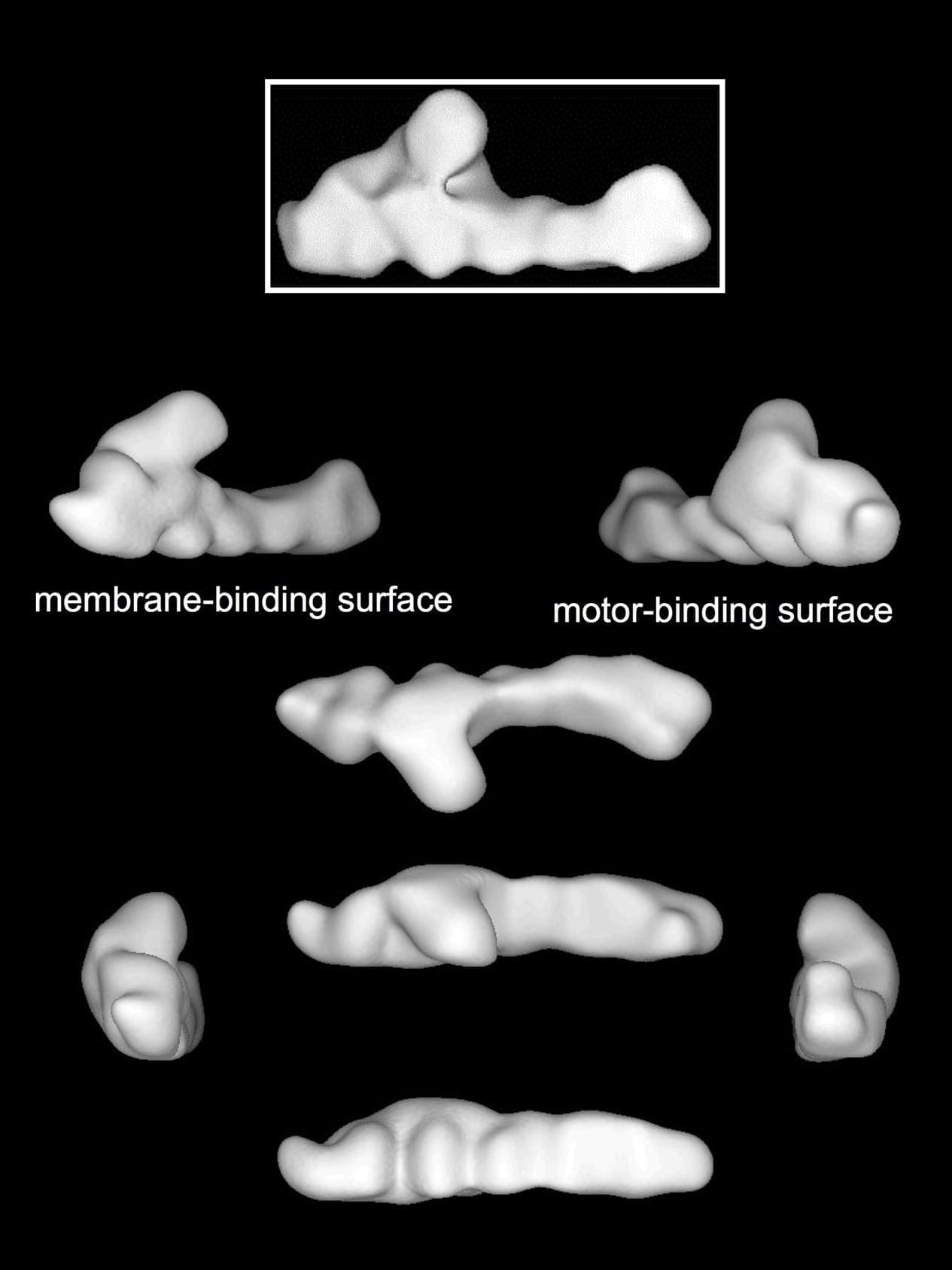
Much of our work is focused on the cytoplasmic dynein motor, specifically its “partner in crime”, dynactin. A multisubunit protein complex with an intriguing shape, dynactin acts as an adaptor that allows dynein to associate with its many subcellular cargoes. Dynein may provide the brawn for movement, but dynactin acts as the brain that controls dynein. Dynactin allows dynein to be targeted to different cellular structures at different times in development and the cell cycle, and also enhances dynein activity directly.
To better understand dynein and dynactin structure and function, we use many approaches, including biochemical, biophysical, molecular biological and microscopy-based techniques. To explore the in vivo functions of these and other molecules, we work in the model systems of fibroblasts, neurons, polarized epithelia and phagocytic cells, each of which allows us to study different aspects of motility. We study subcellular dynamics in real time using sophisticated live cell-imaging techniques.
3D averaged image of dynactin. (Imai et al., in preparation)
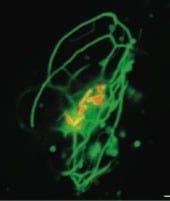
Cells that have been infected with pathogens can provide important new insights into host cell function, and also provide a link to the field of infectious disease. Our work with Salmonella has allowed us to learn more about how intracellular membranes interact with the cytoskeleton and is providing the lab with a fresh view of the molecular mechanisms that control protein trafficking in the endocytic and biosynthetic pathways.
Tubular late endosomes (green) in a Salmonella (red)-infected HeLa cell. (From Drecktrah et al., 2008)
More publications can be found on Google Scholar.
Cavolo, S.L., Zhou, C., Suzuki, M.M., Ukolovic, K, Ketcham, S.A., Schroer, T.A., Silverman, M. A. Levitan, E .S. (2015) Mycalolide B dissociates dynactin and abolishes retrograde axonal transport of dense-core vesicles. Mol. Biol. Cell 26, 2664-2672
Day, C.A., Drake, K.R., Kraft, L.J., Han, B., Davidson, M.W., Holmes, R., Jobling, M.G., Schroer, T.A., Lencer, W.I., Kenworthy, A.K. (2015) Microtubule motors power membrane tubulation in clathrin-independent endocytosis. Traffic 16, 5723-590.
Chowdhury, S., Ketcham, S.A., Schroer, T.A.*, and Lander, G.C. (2015) Structural organization of the dynein-dynactin complex bound to microtubules. Nat. Struct. Mol. Biol., 22, 345-347. PMCID: PMC4385409 (* equal contribution)
Imai, H., Narita, A., Maeda, Y.*, Schroer, T.A.* (2014) Dynactin 3D structure: Implications for assembly and dynein binding. J. Mol. Biol. 426, 3262-3271. (* equal contribution)
Cheong, F.K., Feng, L., Sarkeshik, A., Yates, J.R., Schroer, T.A. (2014) Dynactin integrity depends upon direct binding of dynamitin to Arp1. Mol. Biol. Cell, 25, 2171-2180. PMCID: PMC4091830
Yeh, T.Y., Kowalska, A., Scipioni, B.R., Cheong, F. K., Zheng, M., Derewenda, U., Derewenda, Z.S. and Schroer, T.A., (2013) Dynactin helps target target Polo-like kinase 1 to kinetochores via its left-handed beta-helical p27 subunit, EMBO J. 32, 1023-1035. PMCID: PMC3616283
Blehm, B., Schroer, T.A., Trybus, K., Chemla, Y., and Selvin, P. (2013) In Vivo Optical Trapping Indicates Kinesin's Stall Force is Reduced by Dynein During Intracellular Transport. PNAS 110, 3381-3386.
DeBerg HA, Blehm BH, Sheung J, Thompson AR, Bookwalter CS, Torabi SF, Schroer TA, Berger CL, Lu Y, Trybus KM, Selvin PR (2013) Motor domain phosphorylation modulates kinesin-1 transport. J Biol. Chem. 288, 32612-32621
Wang, S., Ketcham, S., Schon, A., Goodman, B., Wang, Y., Yates, J., Freire, E., Schroer, T.A., Zheng, Y. (2013) Nudel/NudE and Lis1 promote dynein and dynactin interactions in the context of spindle morphogenesis. Mol. Biol. Cell 24, 3522-3533.
Yeh, T.Y., Quintyne, N.J., Scipioni, B.R. Eckley, D.M, and Schroer, T.A. (2012) Dynactin’s pointed end complex is a cargo-targeting module, Mol. Biol. Cell, 23, 3827-3837. PMCID: PMC3459859.
- Muscular Dystrophy Association Postdoctoral Fellowship
- Grass Foundation Fellow
- Searle Scholars Program
- Lucile and David Packard Fellowship for Science and Engineering
- George E. Owen Teaching Award
- American Society for Cell Biology, Women in Cell Biology Junior Award for Excellence in Research
- Award for Excellence in Teaching, Johns Hopkins Alumni Association